Overview of Research Program
The core of our research program is based on the development of new reactions, catalysts and protocols for the synthesis of structurally challenging molecules that exhibit specific biological functions.
Major areas of research interest that we are currently investigating include:
- Development of new methodology for the synthesis of heterocycles for drug-discovery and structurally complex natural products with important biological activity
- New methodologies for the synthesis of amino acids and functionalization of peptides and proteins with the aim of investigating protein-based diseases and developing more efficient biocatalysts
- Development of novel catalytic and flow protocols for efficiently transforming biorenewable terpene and alpha-amino acid feedstocks into fine chemicals, polymers and drugs on an industrial scale
- New types of fluorescent sensor for the selective detection of biologically and environmentally relevant molecules, with a particular interest in imaging biological processes in cellular systems
- Medicinal chemistry programmes for the discovery of new drug molecules that exhibit anti-microbial and anti-inflammatory activities for treatment of infection and auto-immune diseases
All of these research programs have their foundations in organic chemistry, synthesis and chemical biology with strong links with the chemical and pharmaceutical industries, as well as collaborations with numerous academic experts in electrochemistry, biological imaging and medicine at home and around the world.
Synthetic Methodology
Synthetic chemistry is the cornerstone of the natural sciences where it serves as a fundamental technology for the preparation of structurally complex materials on a molecular scale. Complexity in molecules is often associated with chirality - the fundamental property of a structure that renders it non-superimposable on its mirror image. The development of new reactions, catalysts and protocols for the synthesis of chiral molecules is a challenging undertaking and we are particularly interested in developing novel, atom efficient strategies for the asymmetric synthesis of highly desirable chiral fragments for the efficient synthesis of natural product and drug molecule targets.
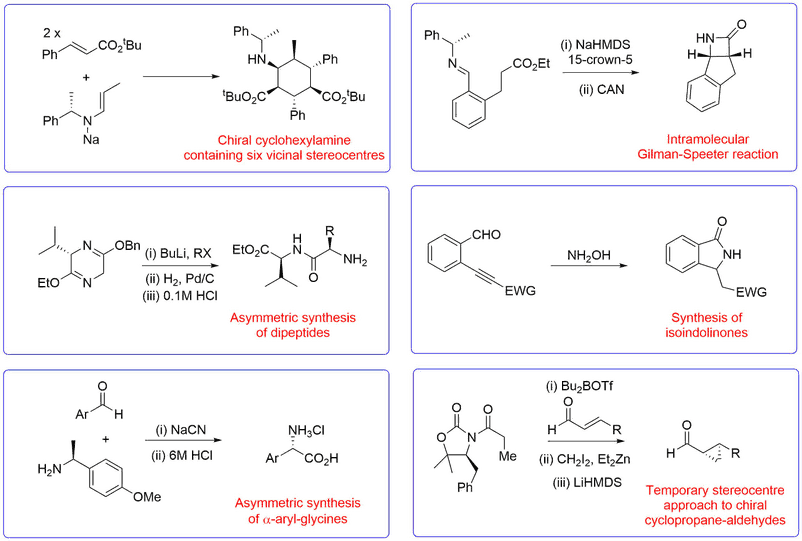
Novel Synthetic Methods
A representative range of new reactions and synthetic methodologies that have been developed in the Bull group for the synthesis of useful chiral fragments of use in natural product synthesis, drug discovery applications and the preparation of peptidomimetics are shown below.
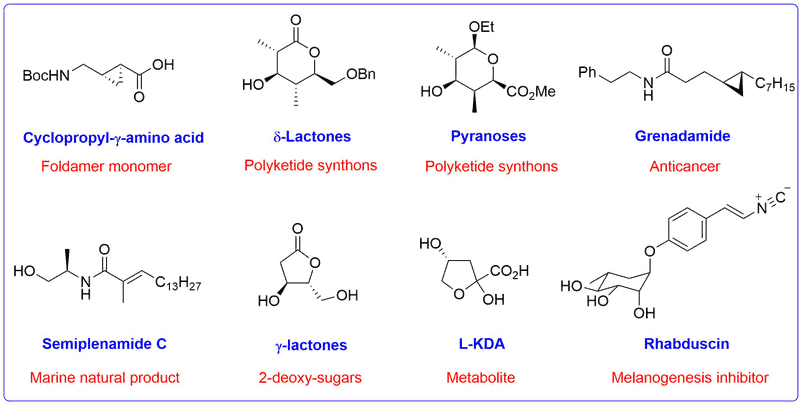
Chiral Synthetic Intermediates and Natural Products
A representative range of chiral targets and natural products that have been prepared using Bull group methodology for their structural or biological activity are shown below.
Catalytic Methodology
There is an increasing realization that many of the synthetic methods in current usage are unsustainable because they employ toxic reagents/solvents, generate large amounts of waste, and consume expensive petrochemicals. Consequently, there is currently great demand for the development of ‘greener’ catalytic protocols that enable the rapid synthesis of existing and new chemical targets of use to the fine-chemical and pharmaceutical industries. Over the last few years, our research group has reported on some significant discoveries using metal catalysts and organocatalysts for synthesis.
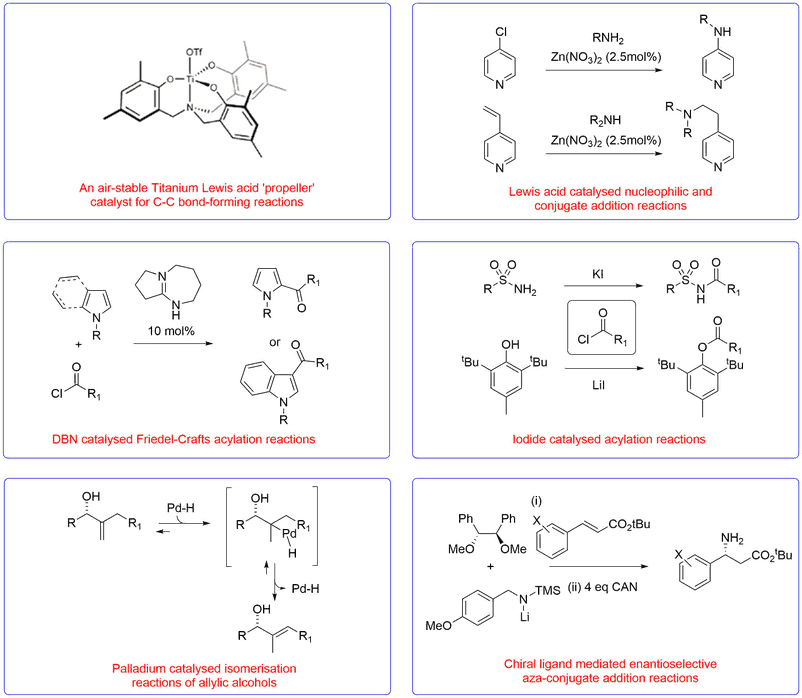
Metal Based Catalysis and Organocatalysis
We have developed a range of novel Lewis acid catalysts, nucleophilic organocatalysts, new reactions and catalytic enantioselective protocols for the synthesis of a range of valuable chemical targets of potential use to medicinal and process chemists for the synthesis of drug molecules.
Biocatalysis
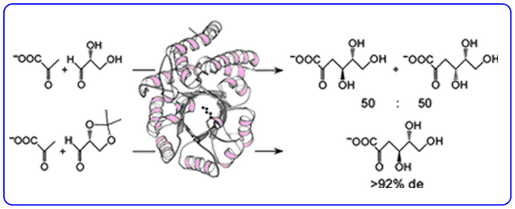
We are interested in determining what strategies enzymes employ to evolve the stereoselectivity of their reactions. Our efforts in chemical biology led to the paradigm discovery of a novel ‘promiscuous’ metabolic pathway in the thermophilic extremophile Sulfolobus solfataricus that has been shown to metabolise both D-glucose and D-galactose using the same series of enzymes.
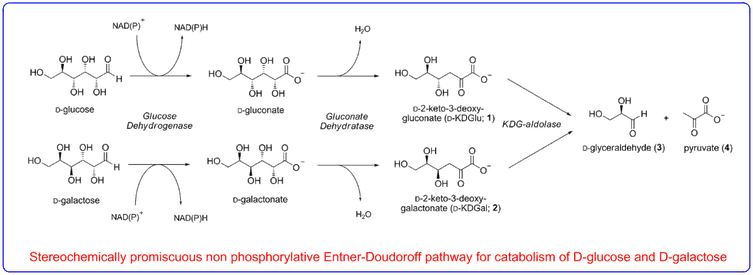
The aldolase of this pathway was shown to catalyse the aldol reaction between its natural substrates pyruvate and D-glyceraldehyde with no stereocontrol to afford a 50 : 50 mixture of D-2-keto-3-deoxy-gluconate (D-KDG) and D-2-keto-3-deoxy-galactonate (D-KDGal) . A substrate engineering strategy using D-glyceraldehyde-acetonide as a structurally rigid substrate was developed to ‘cure’ the stereochemical promiscuity of this aldolase, which enabled D-KDGlu and to be obtained with very high levels of diastereocontrol.
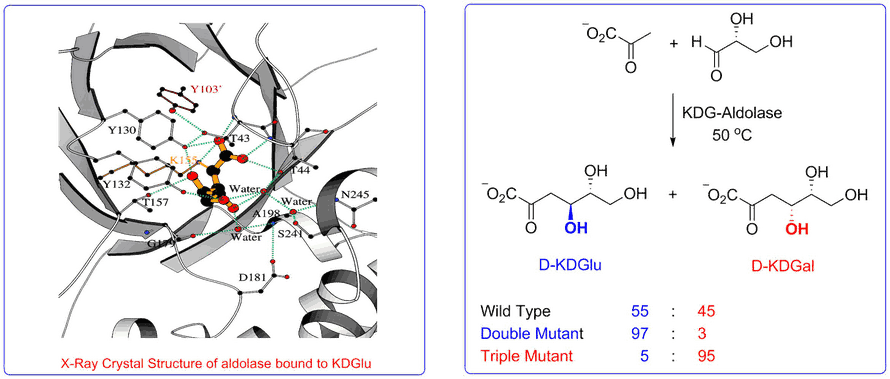
Access to stable X-ray crystal structures of the wild-type-aldolase bound to both D-KDG and D-KDGal enabled us to identify key amino acid residues responsible for binding to each diastereoisomer that enabled structurally informed mutagenesis studies to be used to create aldolase mutants with high diastereoselectivity for the formation of D-KDG and D-KDGal.
Sensor Methodology
We are interested in developing analytical techniques for determining the enantiomeric excess of chiral molecules and for sensing environmentally, biologically and industrially relevant analytes using a range of spectroscopic, fluorescence and electrochemical techniques.
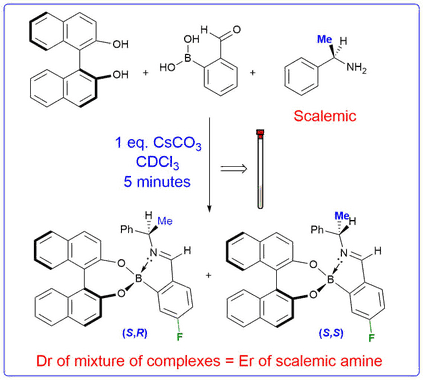
Self Assembling Chiral Derivatizing Agents
We have shown that treatment of a chiral amine and a diol with 2-formylphenylboronic acid results in a novel three-component reaction to afford diastereomeric iminoboronate esters. These complexes exhibit inequivalent resonances in their 1H, 13C and 19F NMR spectra that can be integrated to determine the enantiomeric excess of a parent scalemic amine. The 3-component nature of this chiral derivatisation protocol allows the ee's of chiral diols, amino-diols, diamines, and hydroxylamines to be determined. Alternative strategies based on this self-assembly approach have also been developed that enable the e.e.'s of diols and amines to be determined using cyclic voltammetry and circular dichroism.
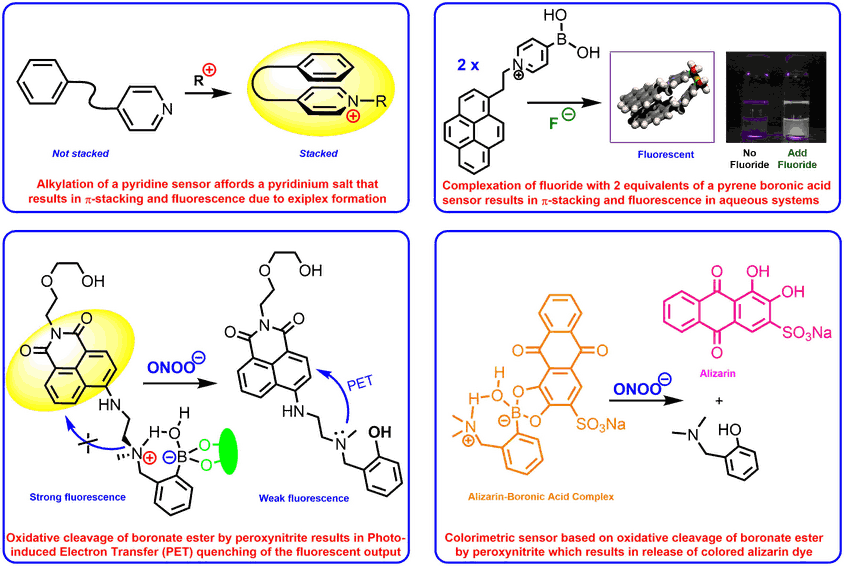
Fluorescent sensors
We have developed a range of fluorescent and colorimetric sensors for the detection of a wide range of analytes, including diols, fluoride, alkylating agents and medicinally important reactive oxygen species such as peroxynitrite or hydroxyl radicals. A number of these sensors have been designed to work in biological systems enabling the presence of analytes to be sensed in real time in cellular systems.
Electrosynthesis
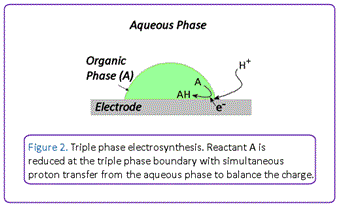
We have been developing highly efficient electrosynthetic protocols that employ electrodes as a source of electrons to facilitate chemical reactions under environmentally benign conditions under triple phase boundary conditions (Figure 2). A triple phase boundary is a junction where an aqueous phase, an organic phase, and a solid carbon electrode meet to provide a localised microreactor where efficient electrochemical transformations can occur. This occurs because substrates can diffuse efficiently to the boundary region where simultaneous electron and ion transfer can occur to maintain charge neutrality. Importantly, this system allows supporting electrolyte and substrates/products to be contained in separate phases, thus avoiding difficulties associated with post-reaction separation of excess electrolyte from products.
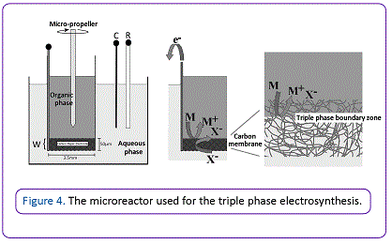
Dynamic variants of triple phase experiments have been achieved using flow chemistry, ultrasonic agitation, and ultra Turrax shear forces to encourage rapid diffusion, with high yields of ion transfer having been achieved using ferrocene based redox systems. Scaled up triple phase boundary microreactors that contains a porous carbon membrane electrode has been developed for the rapid and clean electroreduction of activated olefins, with the electrolyte being contained in a reusable aqueous phase (Figure 4).
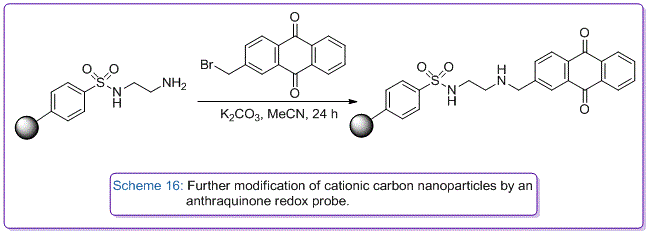
We have also developed synthetic protocols to transform commercially available anionic sulfonate carbon nanoparticles with diamines to create cationic amino-nanoparticles that can be used as binding agents to layer carbon onto glass electrodes to afford large surface area three dimensional catalytic carbon membranes. These modified electrodes have been used to develop solid supported enzyme catalysed reduction reactions of oxygen for use in fuel cells. Further covalent modification of their amino groups, have afforded immobilised anthraquinone nanoparticles as localised pH sensors (Scheme 16), and immobilised boronic acid nanoparticles to be used as electrochemical sugar sensors.
Research Highlights
Some of the Bull group’s research has been highlighted recently in the literature as particularly noteworthy or significant.
- Our research program continues to attract attention worldwide, with Web of Science showing that Steve's publications attracted a H-Index of 50 and >1,000 citations a year for the first time in 2021.

- Our recent joint publication (J. Chem. Ed., 2017, 94, 79-84) on using the Bull-James NMR protocol for determining the enantiomeric excess of chiral amines as an advanced Undergraduate experiment has made the Front-Cover of J. Chem. Education

- In recognition of his contribution to synthetic methodology, Steve was asked to submit a Feature Article on his research into the asymmetric synthesis of alpha-amino acids and dipeptides (Synthesis, 2016, 48, 2036-2049) that ended up on the Front-Cover of Synthesis

- Our research into developing fluorescent sensors for the selective detection of highly reactive peroxynitrite species in-vivo (Chem. Sci., 2014, 5, 3368-3373) was chosen as the front-cover of Chemical Science

- Our research on functionalised carbon nanoparticles as high-surface-area electrode materials in collaboration with Professor Marken's group (Chem. Asian J., 2014, 9, 1226-1241) was highlighted as a Feature Article by Chemistry Asian Journal.
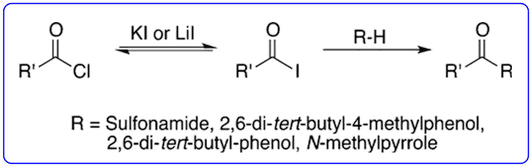
- The ability of iodide salts to catalyse the Friedel-Crafts acylation reactions of acid chlorides with pyrroles and indoles (Org. Lett., 2013, 15, 702-705) was highlighted in ChemInform
- Our work describing the asymmetric tandem hydroboration/reduction of beta-gamma-unsaturated esters to afford chiral diols (Tetrahedron Lett., 2013, 77, 27) was highlighted in ChemInform

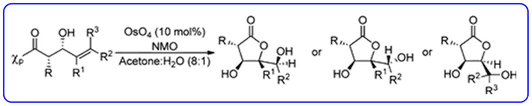
- Our dihydroxylation chemistry for the asymmetric synthesis of highly functionalised chiral gamma-lactones containing multiple stereocentres (J. Org. Chem., 2012, 77 , 543-555) has been highlighted in ChemInform
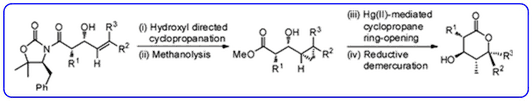
- The diastereoselective synthesis of substituted δ-lactones containing multiple contiguous stereocentres (Org. Lett., 2011, 13, 3592-3595) has been highlighted in Synfacts.
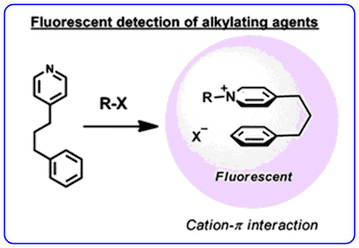
- The recent publication describing the development of an alkyl halide sensor using cation-π interactions (Chem. Commun., 2011, 47, 253-255) has been highlighted as Noteworthy Chemistry by Chemistry and Engineering News.
- Our work on air-stable titanium triflate as a Lewis acid catalysts for Diels-Alder reactions and other carbon-carbon bond forming reactions (Chem Asian J., 2010, 5, 612-620) has been highlighted in Cheminform
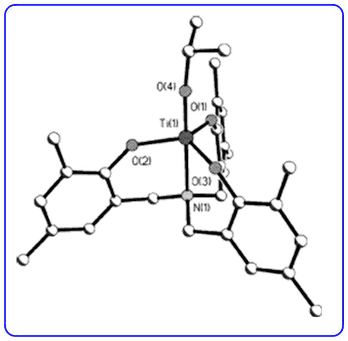
- The diastereoselective three-step synthesis of highly substituted γ-butyrolactones (Org. Lett., 2009, 11, 2896-2899) has been highlighted in Synfacts.
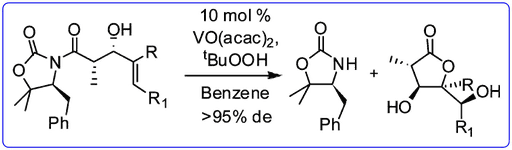
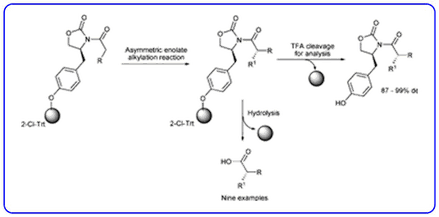
- The enolate alkylation reactions of polymer supported Evan’s auxiliary (Chem. Commun., 2008, 508-510) has been highlighted in Synfacts.
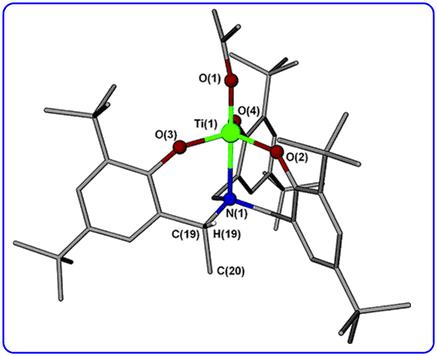
The synthesis of a titanium alkoxide with propeller-like chirality (See Org. Lett., 2007, 9, 223-226) was highlighted in the Editors’ Choice column in Science.
.
Page maintained by Dr Robin Groleau ·
Last updated: October 2021 © 2021 · Disclaimer · Privacy Statement